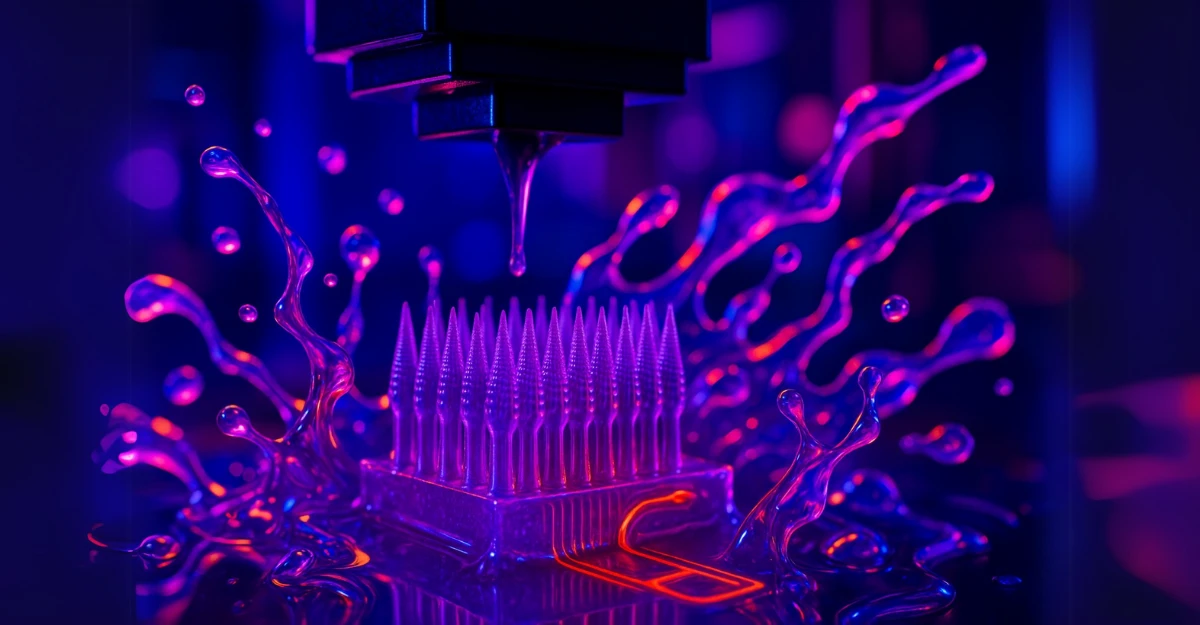
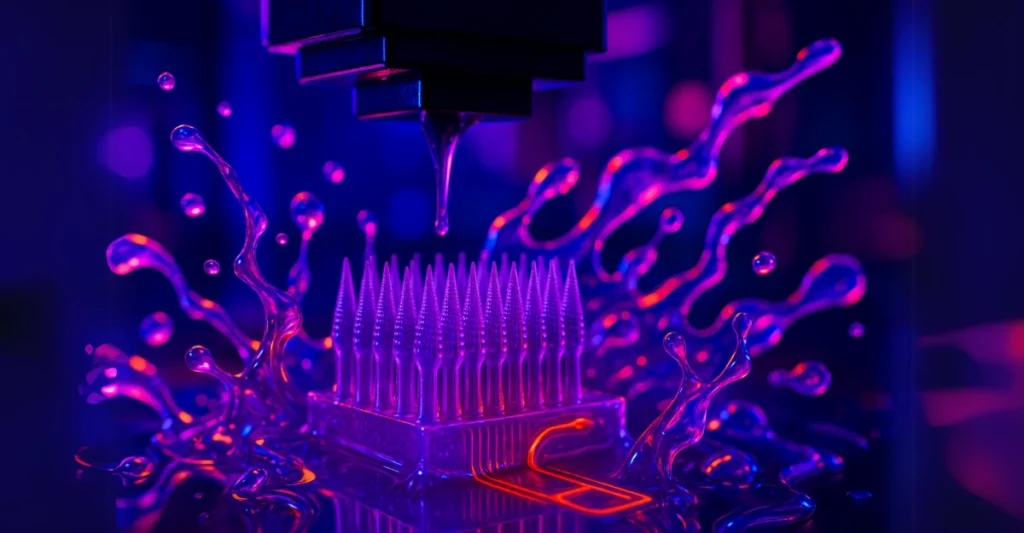
Continuity Biosciences bets on PinPrint’s unproven-but-promising 3D microneedle tech for cosmetic and therapeutic applications.
A recent investment by US biotech firm Continuity Biosciences into startup PinPrint suggests the companies are betting big on 3D printed microneedle technology though whether it lives up to the hype remains to be seen.
The deal, announced this week but with financial details conspicuously absent, would see Continuity expand its focus from implanted drug delivery systems to PinPrint’s experimental microneedle patches. These patches, still in development, claim to deliver vaccines and cosmetic agents intradermally without traditional injections. If proven effective, they could offer a pain-free alternative but that’s still a big “if.”

PinPrint’s approach relies on a proprietary 3D printing method called iCLIP, co-developed by Dr. Joseph DeSimone, the controversial former CEO of Carbon. The technology allegedly solves resin over-curing issues that plague microfluidic device production, though independent verification of these claims is sparse. A 2023 paper in PNAS detailed the method, but real-world applications remain untested at scale.
“This investment lets us explore non-implanted delivery platforms,” said Continuity CEO Dr. Ramakrishna Venugopalan, who will join PinPrint’s board. The wording is carefully optimistic acknowledging this is more exploratory than guaranteed success.
The Unproven Promise of iCLIP
PinPrint’s iCLIP method supposedly works by continuously feeding fresh resin during printing to prevent distortion in microchannels critical for microneedle precision. Lab tests show promise, but manufacturing consistency and regulatory hurdles for medical use are unanswered questions.
Also read: Elegoo Holds Centauri Carbon Price Despite Tariff Hikes
Meanwhile, competitors like CADworks3D and Microlight3D are taking different approaches to microfluidics, suggesting the industry isn’t sold on a single solution yet. PinPrint’s edge, if it exists, hinges on DeSimone’s reputation and early academic results not commercial validation.

A Waiting Game
With microneedles still a niche technology and 3D printing’s role in mass medical production unproven, Continuity’s investment is either prescient or premature. The partnership hints at a future where drug delivery is painless and personalized but for now, it’s still just a leaky rumor of potential.